

Prospective Study of Autologous Adipose Derived Stromal Vascular Fraction Containing Stem Cells for the Treatment of Knee Osteoarthritis
Mark Berman, MD*, Elliot Lander, MD, Thomas Grogan, MD, Walter O’Brien, MD, Jonathan Braslow, MD, Shawntae Dowell and Sean Berman, MS
Abstract
Background: The management of osteoarthritis of the knee runs the spectrum of care from a variety of conservative treatments often culminating in total joint arthroplasty. We initiated a large prospective study to evaluate whether autologous adipose derived stromal vascular fraction (SVF rich in stem cells) therapy is a safe and effective option.
Methods: A patient funded prospective study of 2,586 patients from a network of physicians participated in an IRB approved study using autologous stromal vascular fraction SVF (ClinicalTrials.gov as NCT10953523). All patients were treated with a standardized surgical protocol to harvest lipoaspirate, isolate and deploy autologous SVF. Data was collected using an online registry and patients were followed-up via an automatic online database collection service.
Results: 2,586 patients were treated. Statistically significant improvement was seen at 1 and 2 years – meaning less pain and greater ease of mobility. There was no differ- ence between male or female outcomes (82% overall improvement). All BMI levels showed improvements though higher BMIs had less improvement. There was no difference in outcomes between SVF alone or with PRP added to SVF. Improvement was the same regardless of payment or receiving free care. There were very few adverse events and those that did occur were largely very minor or easily treatable.
Conclusion: Deployment of autologous SVF represents a simple surgical procedure that can be safely performed in an adequate outpatient environment under straight local anesthesia and demonstrates very good outcomes even in difficult cases of chronic knee arthritis.
Introduction
Multiple peer-reviewed publications exist showing that adipose derived stromal vascular fraction containing adult mesenchymal stem cells may improve the con- dition of inflammatory knee conditions.
Animal studies show double-blind examples of significant improvements in using adipose derived stem cells for degeneration and injuries.
Multiple studies suggest that allogeneic stem cells may be efficacious and safe. While an off-the- shelf stem cell product might be idyllic, there are still long-term concerns about immunogenicity as allogeneic cells, while initially immune neutral, or immune evasive, will differentiate into the donor immune char- acteristics. Autologous stem cells possess no risk of short or long-term allergic or immune response. Additionally, an allogeneic source of human stem cells must be free of any infectious agents. As humans are not grown in sterile environments, it’s virtually impossi- ble to assure society that allogeneic sources are devoid of possible prions or viruses that may go undetected. A variety of papers have already documented risks of dis- ease transmission from a variety of allogeneic sources.
Patients presenting with painful knee osteoarthritis may benefit from receiving immediate point of adipose stromal vascular fraction (SVF) surgically isolated from lipoaspirate. At the time of treatment, patients may also have the option of sending their autologous adipose tissues to a laboratory banking facility for cGMP production and storage of Mesenchymal Stem Cells (MSCs) for future use or repeat treatments. Yet, same day treatments with autologous cells (SVF) prevents the necessary wait time for cGMP laboratory preparation.
In order to evaluate the efficacy and safety of adipose-derived SVF for arthritic knee pain, an IRB approved outcomes study was initiated in 2012 through to 2015 using a standardized surgical procedure among a number of different Cell Surgical Network affiliated medical clinics that share protocols and techniques for SVF production and deployment. There was no placebo arm using sham surgery. Patients were queried via an automated email campaign to ascertain clinical out- comes following intravenous and intra-articular deployment of adipose-derived SVF. The initial goal was to learn if autologous SVF delivered using a simple point of care surgical procedure was safe and effective. Results were stratified and analyzed to determine optimal conditions.
Materials and Methods
An IRB approved (International Cell Surgical Society) outcomes study and IRB approved consent for os- teoarthritis, including knees, was initiated in 2012 and registered with ClinicalTrials.gov as NCT10953523. Patients over 18 years of age that had complaints of knee arthritis were considered for therapy. Patients were not limited to any particular Kellgren-Lawrence scale. Patients with active systemic infections or dental infec- tions were excluded. Most patients had already tried and failed to improve with a variety of interventions in- cluding NSAIDS, steroid injections, platelet rich plasma injections, rest, heat, cold, magnetic devices, hyalurons and even arthroscopic procedures- none of which pro- vided sustained relief beyond a few weeks resulting in reduced mobility. Data was collected from all participat- ing affiliates of the Cell Surgical Network (CSN) through an HIPAA compliant online database (TrackVia.com). CSN affiliate sub-investigators that contributed to this study are listed in the acknowledgement section.
CSN affiliates who were not orthopedic specialists themselves were encouraged to work in multi-disci- plinary teams in order to have an orthopedic or sports medicine specialist involved in the care and follow-up of the patients. Many affiliates from other specialty back- ground, such as pain management or family practice, etc., by virtue of their education, training and experience, felt qualified evaluating, treating and following the patients themselves.
All patients underwent a uniform mini-liposuction procedure consistent with the CSN IRB approved protocol. This included a specific method for providing a sub-dermal local anesthesia to maximize comfort, safety, and limit exposure of stem cells to potentially cytotoxic levels of lidocaine. Syringe lipo-aspiration was accomplished using specially developed syringes (Medikan, South Korea). 50 ml of lipoaspirate was recov- ered from one of several potential patient harvest sites. The site depended on a variety of issues doctor preference, patient preference, cosmetic result, consideration for possibly more optimal stem cell sites and ultimately the abundance of harvestable adipose tissue.
The lipoaspirate was condensed by centrifugation (2,800 rpm for 3 minutes) and the infranatant cells (ap- proximately 1-2 ml because a number of cells are al- ready mechanically dissociated) and approximately up to 25 ml of condensed fat were transferred to another specialized syringe (TP102 Medikan, South Korea) along with 25 ml of 12.5 Wunsch units of GMP collagenase (Roche) in normal saline for incubation with gentle ag- itation at 37 °C for 30-40 minutes. Following this, the lipoaspirate was centrifuged for 4 minutes at 200 rcf (relative centrifugal force approximately 1,100 rpm). With unique equipment, the supranatant fluids were removed while maintaining the infranatant solution within the syringe. This prevented air exposure to the SVF preparation and allowed introduction of D5LR in order to dilute the collagenase by washing to imperceptible levels. Three dilutions were completed leaving 4-10 ml of concentrated cells at the bottom of the syringe. This solution was transferred to a 10 ml luer lock syringe and filtered through a 100-micron nylon filter producing 4-10 ml of final SVF isolate.
The affected knee or knees were injected with 3-5 ml of SVF solution. This was most frequently done by injection either medially or laterally just above the tibial plateau and deep into the joint. A wheal of local anesthesia or ethyl chloride spray was provided for skin anesthesia. A 22 g, 1.5 inch needle was most often used for the deployment into the joint. Most physicians performed the procedure in the office or surgical treatment room without aid, however, in some cases, ultrasound or fluoroscopy were employed. Most often the remaining solution was provided via an intravenous infusion. In some cases, doctors chose to add Platelet Rich Plasma (PRP) to the intra-articular deployment. There have been a plethora of studies suggesting PRP brings a variety of important growth factors to the injury and may complement the addition of SVF. Physicians and patients were allowed to make the decision whether or not they wanted to add PRP and results were appropriately stratified.
Due to the high number of patients included in this particular study, responses to the WOMAC questionnaire proved futile. Questionnaire response rates improved significantly when patients recorded a simple visual acuity pain score of 0 (no pain) to 10 (worse pain they could tolerate) for 1) At rest, 2) Standing, 3)Walking and 4) Running. An automatic program through Trackvia (Denver, CO) to contact and query our patients for follow-up responses at intervals of 1 week, 3 months, and then every 3 months until 36 months (3 years) and the yearly for the next two (4 and 5) years was used. Patients were asked to note their visual acuity pain scores each time and provide an overall opinion as to whether they had sustained improvement in function (i.e. mobility) without or with decreased or tolera- ble pain compared to before the received deployment. Pain scores are reasonably objective while improvement based upon patient response remains subjective.
Prior to SVF deployment, sub-investigators mea- sured total cell counts using the Countess device (Invitrogen) that quantified cells over 10 microns in size (to exclude RBCs). Results ranged from 10 to 300 hundred million cells. Viability was also checked using 0.4% trypan blue and ranged from 65-95%. Cell counts varied significantly among patients as expected with an autologous biologic. SVF cannot currently be isolated at point of care uniform in dose, strength and purity due to the wide variety and proportion of cells in the final isolate. Stem cell counts were not obtained on study patients however as pre-clinical work, 50 SVF samples were sent for flow cytometry to a collaborative research lab (i.e. Stem Immune) at UC San Diego and large but variable quantities of several types of stem cells were identified in each sample.
Flow cytometry analysis showed that freshly isolated SVF was heterogeneous and harbored four major sub- sets specific to adipose tissue:
- CD34 high, CD45-, CD31-, CD146- adipose-derived stromal/stem cells (ADSCs),
- CD34 low, CD45+, CD206+, CD31-, CD146- hema-topoietic (or angiogenic) stem cell-progenitors (HSC-progenitors),
- CD34 high, CD45-, CD31+, CD146+ adipose tissue-en- dothelial cells, and
- CD45-, CD34-, CD31-, CD146+ pericytes.
Statistics were determined using the ANOVA method.
Table 1: When queried whether the patient experienced improvement (measured as a decrease in pain or improvement in perceived function mobility versus no improvement in pain or mobility) the above graph revealed the following:
Baseline total – 2,586 patients
- 1 month – 1,643; Improve – 1,150 (69.99%); Not improve – 408
- 3 month – 1,330; Improve – 1,027 (77%); Not improve – 226
- 6 month – 1,097; Improve – 862 (78.58%); Not improve – 154
- 9 month – 803; Improve – 642 (79.95%); Not improve – 96
- 5. 1 year – 615; Improve – 495; (80.49%); Not improve – 69
- 1 year 3 months – 363; Improve 293 (80.72%); Not improve – 34
- 1 year 6 months – 248; Improve – 198 (79.84%); Not improve – 25
- 2 years – 212; Improve – 165 (77.83%); Not improve – 23
- 3 years – 57; Improve – 47 (82.46%); Not improve – 7
- 4 years – 15; Improve – 13 (86.67%); Not improve – 1
- 5 years – 4; Improve – 4 (100%); Not improve – 0
Results
2,586 patients participated in SVF deployment. There was a drop-off in data collection even at one month to 1,643 patients. Overall, patients reported that they had improvement with pain or function in nearly 80% of the cases. There were a few patients reporting 4 and 5 years later that they still maintained good results, but these numbers were obviously few (See Table 1 above). There were statistically significant improvements at 1 and 2 years respectively based upon n = 615 and n = 212 patients reporting. Improvement was determined subjectively by patients both based upon actual decrease in pain and their impression of whether they improved with respect to pain and function (i.e. mobility). A typical standing knee x-ray often revealed a lack of joint space and with improvement, an increase in joint space was often seen suggesting possible cartilage re-growth (Figure 1). The study stratified the patients and reported re- sults based upon averages among all patients. Many patients were restored to pain free activities. Many younger patients were able to resume complete com- petitive sporting activities, while many older patients were content to be able to walk without pain so they could resume travel, golf and normal daily routines.
Figure 1
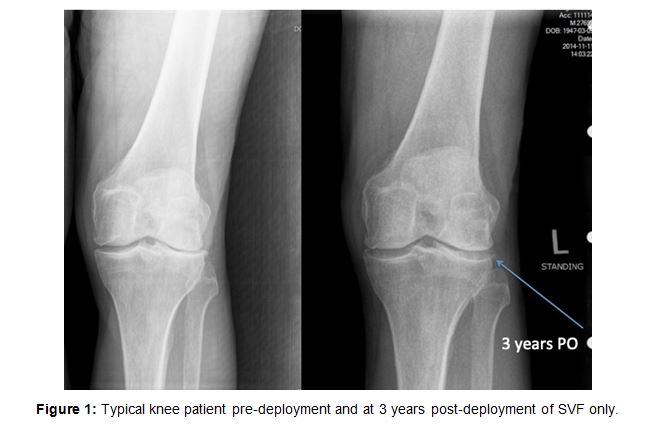
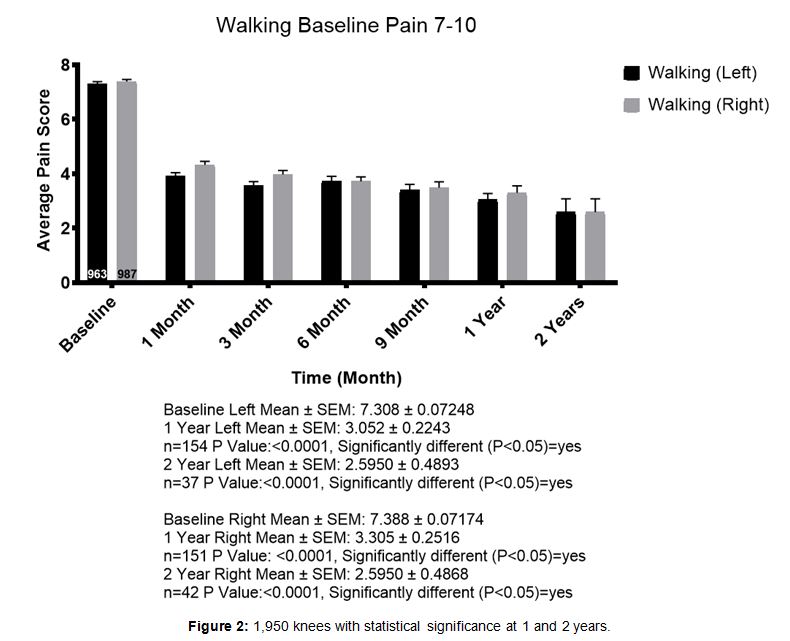
Patients were stratified into groups according to their pain level as 7-10 or 1-6. 1,017 patients were en- tered in the 7-10 pain level for walking. Figures rep- resent patients followed for walking pain up to two years. Mean baseline pain level for the left and right knee respectively, was 7.308 and 7.388. At one year, 154 left knee patients reported pain levels at an average of 3.052 and 151 right knee patients reported pain levels averaging 3.305. This was considered sta- tistically significant (Figure 2).
Figure 2
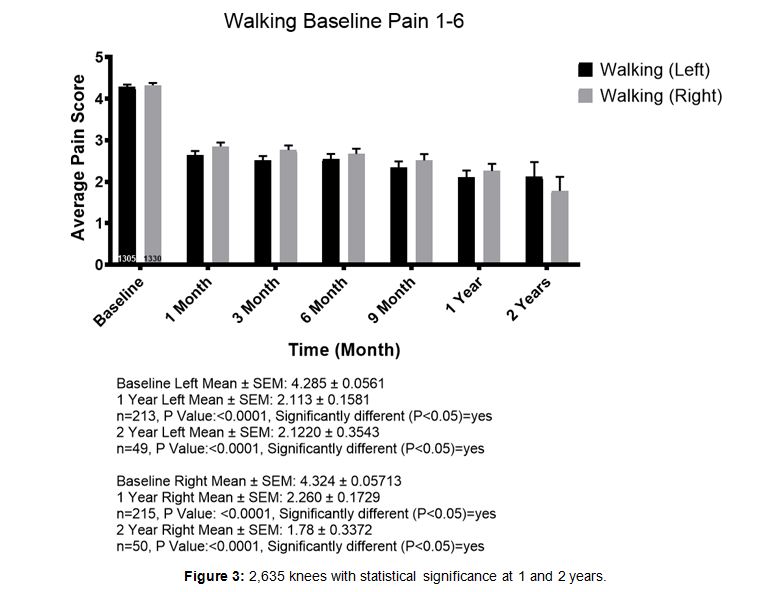
1,404 patients were entered into the 1-6 pain cate- gory for walking. Their mean baseline pain level for the left and right knee respectively, was 4.285 and 4.324. At one year, 213 left knee patients reported an average of 2.113 and 215 right knee patients reported pain levels averaging 2.260. This was considered statistically significant (Figure 3). All age groups (from below 30 to over80) posted results consistent with the overall trends.
There did not appear to be any age category that did better or worse than another. In general, most patients started with walking pain in the 4-5 range on average and after 1 year the average of 1-2 pain score for walking was seen across all age categories.
There was no significant difference between the overall results between men (n = 1,907 knees) and women (n = 1,848 knees). Both reported improved results just above 82% on average for all times reporting, with statistically significant results at 1 and 2 years.
Figure 3
When patients were stratified by body mass index (BMI) there was improvement for most patients in all categories. Patients with higher BMI, particularly over 30, had less improvement compared to lower BMI patients, yet still reported improvements on average. While the trends were the same in all categories, we demonstrate BMI 18-25 (i.e. normal range) compared to BMI 30+ (very overweight or obese) (Figure 4a and Figure 4b).
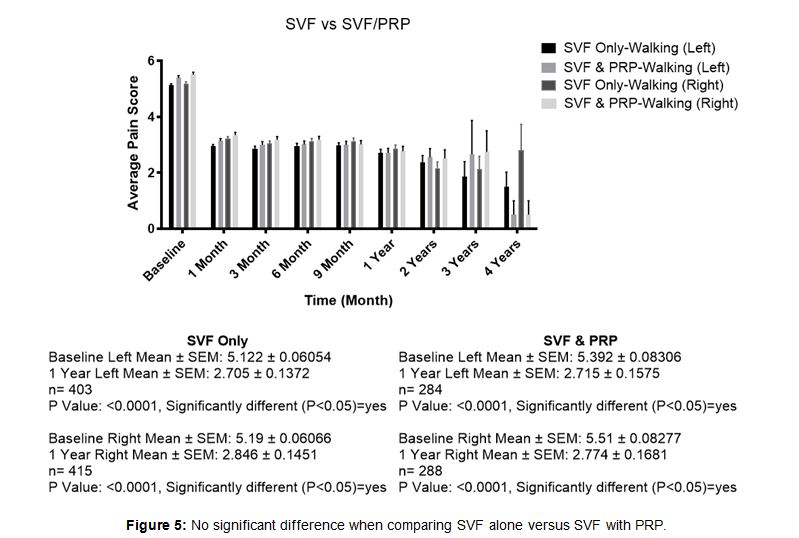
Some investigating physicians used PRP as a supple- ment to SVF injected into the knee joint. Comparison of SVF alone to SVF supplemented with PRP revealed no significant difference in the overall results at the end of one year. Nearly all patients in both of these cate- gories received supplemental IV infusion ( Figure 5). SVF patients (n = 818 experienced a baseline walking average pain of 5.17 and a 1-year walking pain decreased to an average of 2.75. SVF and PRP combined patients (n= 572) experienced a baseline walking average pain of 5.35 and a 1-year walking pain decreased to an average of 2.74.
Figure 4a
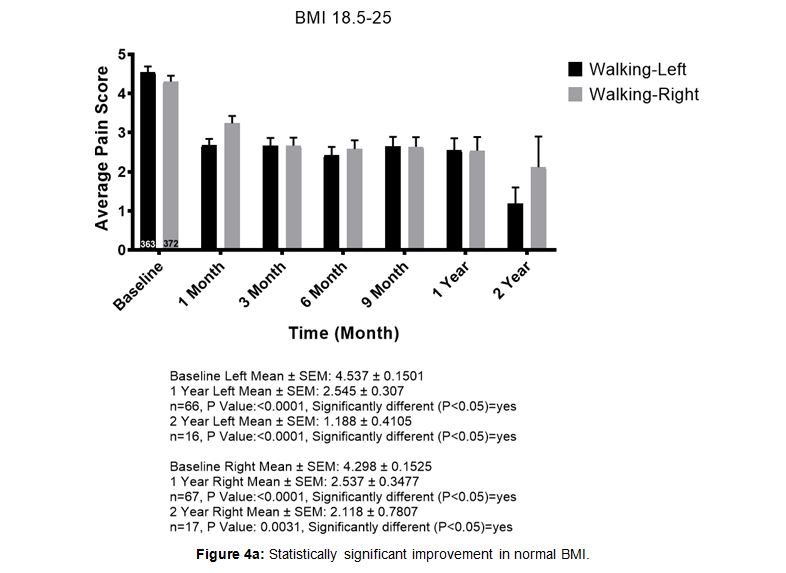
Figure 4b
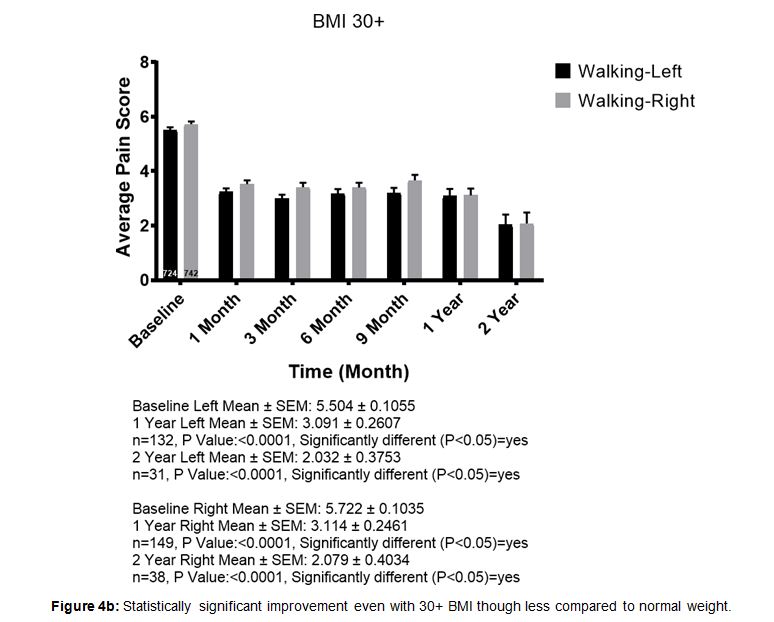
Figure 5
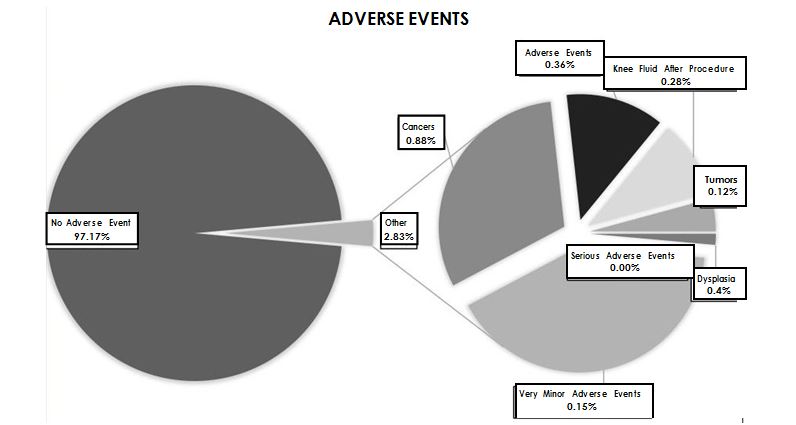

There were no serious adverse events such as death, hospitalization, infections, pulmonary emboli or other serious thrombus formation. A few patients thought they had increased pain and about 18% of the overall patients did not report improvement. Lack of improvement may be a risk but not an adverse event. A number of these patients went on to have total knee replacement. We do not have specific sta- tistics on their outcomes following total arthroplasty. The most common problem was near immediate knee swelling and pain (0.28%). When this occurred there was never a positive culture and whenever steroids were provided (dexamethasone 4-10 mg given as soon as possible into the tender edematous joint), there was a uniformly positive response. All cases had favorable responses and the patients went on to have good improvement without needing further treatments. One patient erroneously reported that they sustained a serious knee infection and eventually needed a total arthroplasty. The non-CSN affiliated physician that saw her the following day unfortunate- ly drained the patient’s knee without providing steroids, sent the specimen for culture that proved to be negative and injected the joint with bupivacaine
In assessing possible tumor or cancer formation, few patients responded positively that they had cancers or benign tumors over a course of time. Most cancers or tumors occurred more than 18 months following the procedure. Some lesions, particularly skin cancers, had previously occurred and were reported months later oc- curring again. None of the tumors or cancers were considered to have been caused by the SVF and nor did they occur in any frequency greater than would be expected in the general population.
All other adverse events were not considered note- worthy.
Cell counts were done on all specimens collected however they weren’t correlated with flow cytometry so there was no way of gauging any value in the data. When cells were counted using either size (e.g. cells between 10 and 60 micron) or structural elements (e.g. nucleated cells) to exclude red blood cells, counts varied from 30 to 800 million cells overall. Most cell counts ran around 50 – 100 million cells overall. Evaluation by flow cytometry of 50 samples of cells suggested that stem cell populations were approximately 10% of our overall cell count, but this didn’t appear to be statistically significant. Overall cell viabilities ran between 70 and 90% remaining fairly constant throughout the data collection.
Discussion
By working together as a clinical research network, using the same equipment, same methods of anesthesia and surgical preparation, data was gathered from over 80 independent clinical sites affiliated with the Cell Surgical Network. Most clinics worked with a multispecialty team that included orthopedic surgeons. Patients self reported subjective outcomes data into the online HIPAA compliant database directly allowing the network to collect safety and efficacy data from all clinics. A significant amount of information collected through the database and analyzed demonstrated relative uniformi- ty throughout the network. These CSN physician teams deserve considerably more gratitude and acknowledgment than the simple table we have included below in the Acknowledgement section.
As previously noted in safety studies, SVF is shown to be exceedingly safe. This knee study exceeds the number of patients analyzed in previous safety studies and certainly corroborates the safety of autologous SVF for deployment to the knees along with an IV infusion. Nearly all of the 2,586 patients received supplemental intravenous infusions and IV infusion is currently considered part of the best practices protocol.
There were no serious adverse events (e.g. death, hospitalization, emboli, infections from SVF, etc.) directly related to receiving SVF. There were some adverse events reported by the patients with the most notable being related to knee swelling within 24 hours of the procedure. In some cases it was quite painful, but in all cases, when treated with an injection of dexamethasone 4 mg intra-articular, it quickly resolved (within minutes or hours). None of them were infected (no positive cultures). Most adverse events were related to pain around the liposuction site and that was generally minimal (responded to temporary pain medication) and transient in all cases. The reported incidence of cancer or benign tumors generally occurred much later than one would expect in a causal relationship or previously existed (e.g. skin cancers) prior to treatments. The incidence of cancers was not any greater than would be expected in this general population and would therefore not be attribut- able to autologous SVF.
These prospective surgical procedures were done as part of an Institutional Review Board (organized through the International Cell Surgical Society) approved study. As a surgical procedure it was important to gather data to look for safety as well as efficacy. As a surgical procedure with an abundance of publications suggesting efficacy, a patient registry was established for prospec- tive data rather than trying to set up a placebo trial. The amount of statistically significant data suggests that SVF is efficacious for knee arthritis amongst a variety of patients. Fairly uniform results were observed across all age groups. Patients with BMI above 30 faired less well than those below 30 however, all groups showed overall positive responses and decreasing pain scores. There was no significant difference between men and women.
When comparing clinical outcomes where PRP was added to the SVF versus SVF deployment alone, no sta- tistical differences were observed in related pain out- comes. SVF is a combination of many cells including platelets, growth factors, and extracellular matrix materials. At least four varieties of adult stem cells have been identified in SVF with the two largest groups noted by CD34 and CD45 markers. Respectively, these represent preadipocytes and angiogenic cells found within the lining of the larger blood vessels.
Patients exhibited a positive response to SVF clinically for what appeared to be fairly long-term compared to other currently available injectable remedies. Most of the patients in this study presented for SVF deployment prior to requiring a total knee replacement procedure. The majority had exhausted all conventional treatments for knee arthritis such as NSAIDs, steroid injections, platelet rich plasma, hyaluronic acid injections, and even arthroscopic intervention. Since there was no lasting improvement from any of these previous treatments, it is suggested that none of the patients had any lasting affects from a possible placebo effect of those treatments. Since most (over 80%) of the SVF treated patients sustained significant lasting improvement (greater than 1 year), it could be suggested that SVF may have a (much) better placebo effect than any of the other previous treatments.
Several of the patients continue to experience posi- tive results as long as 8 years after their initial SVF injection (many prior to the official start of the study group). Since the patients are made up of cells and will continue to be exposed to normal cellular degeneration, the suggestion remains that repeated cell therapy should likely be necessary as time and natural degeneration or arthritic disease progression goes on.
Patients were either called for follow-up or in most cases contacted automatically via a software follow-up program securely integrated with the database. Patient responses were made in the comfort of their own environment without a physician looking over their shoulder or possibly being able to influence their response (i.e. Hawthorne effect). Nonetheless, patient compliance in responding decreased over time. 2,586 patients were enrolled in the study, but only 1,643 responded at the first month and 615 pa- tients at the end of one year. Still, the percentage of patients showing improvement remained relatively stable with a statistical significance.
In a few cases where patients did not respond positively, arthroscopy was performed to evaluate their condition. In each case, at least one of the articular surfaces was devoid of any cartilage. For this reason, it is suspected that if there is no cartilage remaining on an articular surface, then there will be no adequate cellular signals to prompt the SVF (i.e. stem cells) to initiate repair of the arthritic condition.
It is important to note that the patients in the study were routinely charged for their surgical procedure. In many cases, patients were treated at no cost or markedly reduced cost depending upon any number of personal circumstances. This study had no investment or grant funding and as such patient funding was relied upon in order to subsidize those with less economic means. No difference was seen in response between patients that paid for their proce- dure versus those that paid nothing or had reduced fees.
Analysis of the study’s patient population showed that positive results from SVF deployment were largely dependent upon the state of the injury. Most of the patients in the study opted to receive SVF in hopes of avoiding total joint replacement and thus, they exhib- ited chronic conditions that left them with very little or no cartilage(apparent by x-ray) in their joint. Increased resolution of pain and cartilage repair should be achieved by providing cell therapy for patients during an earlier acute phase rather than the more chronic advanced phases of their condition, common in this study. Early on, there is likely to be more car- tilage present, a strong and pronounced cytokine response, and potentially a requirement for fewer stem cells and reduced time for healing.
The SVF isolation procedure is a simple surgical procedure whereupon physicians isolate autologous cells from adipose tissue both through mechanical disruption (e.g. liposuction alone causes disruption of cells from adipose [21]) and other advanced means (e.g. enzymatic digestion of collagen to further free cells from adipose tissue), then redeploy these isolated cells into damaged or diseased tissues during a point of care procedure. This surgical method of performing “personal cell therapy” (PCT) provides the physician with an advanced alternative method to care for patients with arthritic conditions. Many current therapeutic endeavors are aimed at mitigat- ing symptomatic pain or reducing the secondary inflammatory response (i.e. prostaglandin formation) to limit pain in hopes of giving the body time to “heal its injury.” By initially providing SVF, rich in stem cells, it is likely that the healing cells will respond to the cytokine response from the injury in order to effect primary healing. With new technology, there typically comes skepticism from both patients and referring physicians. As such, it’s understandable that the majority of patients currently seeking investigative personal cell therapy will exhibit later stage chronic conditions that are more difficult to repair than those treated closer to the acute phase. Ultimately, as cell therapy gains widespread acceptance, it is likely that there will be a greater tendency toward cell deploy- ment closer to the acute phase of injury in order to achieve more optimal results. Read full article.
Adipose – Derived Stem Cell Therapy Research
In 2001, researchers at the University of California, Los Angeles, described the isolation of a new population of adult stem cells from liposuctioned adipose tissue. These stem cells, now known as adipose-derived stem cells or ADSCs, have gone on to become one of the most popular adult stem cells populations in the fields of stem cell research and regenerative medicine. As of today, thousands of research and clinical articles have been published using ASCs, describing their possible pluripotency in vitro, their uses in regenerative animal models, and their application to the clinic. This paper outlines the progress made in the ASC field since their initial description in 2001, describing their mesodermal, ectodermal, and endodermal potentials both in vitro and in vivo, their use in mediating inflammation and vascularization during tissue regeneration, and their potential for reprogramming into induced pluripotent cells. Read full peer reviewed article
Most people would be happy to get rid of excess body fat. Even better: Trade the spare tire for something useful — say, better-functioning knees or hips, or a fix for an ailing heart or a broken bone.
The idea is not far-fetched, some scientists say. Researchers worldwide are repurposing discarded fat to repair body parts damaged by injury, disease or age. Recent studies in lab animals and humans show that the much-maligned material can be a source of cells useful for treating a wide range of ills.
At the University of Pittsburgh, bioengineer Rocky Tuan and colleagues extract buckets full of yellow fat from volunteers’ bellies and thighs and turn the liposuctioned material into tissue that resembles shock-absorbing cartilage. If the cartilage works as well in people as it has in animals, Tuan’s approach might someday offer a kind of self-repair for osteoarthritis, the painful degeneration of cartilage in the joints. He’s also using fat cells to grow replacement parts for the tendons and ligaments that support the joints.
Foremost among fat’s virtues is its richness of stem cells, which have the ability to divide and grow into a wide variety of tissue types. Fat stem cells, also known as adipose-derived stem cells, can be coerced to grow into bone, cartilage, muscle tissue or, of course, more fat.
Multipurposes
Cells from fat are being tested to mend tissues found in damaged joints, hearts and muscle, and to regrow bone and heal wounds.
The stem cells in fat share the medical-worthy spotlight with a few other cells. Along with the fat-filled adipocytes that store energy, fat tissue has its own blood supply and supporting connective tissue, called stroma. The stroma contains blood cells, immune cells, endothelial cells that line the inner surface of blood vessels and pericytes, which line the outer surface. These other fat-derived cells are proving to have therapeutic value as well.
Plastic surgeon J. Peter Rubin, also at Pitt, says that the multitalented cells found in fat could prove to be the ultimate body repair kit, providing replacement tissue or inspiring repair of body parts that can’t mend themselves.
Much of the research — more than a decade of studies — has been in lab animals, but a few applications are being tested in human volunteers. Current clinical studies under way aim to provide replacement tissue to treat chronic wounds and diabetic sores, or conditions such as Parkinson’s disease, multiple sclerosis, chronic obstructive pulmonary disease and type 1 diabetes.
Most clinical studies use the simplest approach: Harvest cells from a patient, then inject them in a single procedure. In more complex approaches still in lab and animal testing, various cells in fat are extracted and manipulated to create custom treatments for worn-out or damaged tissues or to generate blood flow after a heart attack or replace bone in large fractures.
Questions remain, however, about how the cells do their regenerative magic. Scientists and regulators still have plenty to figure out, such as what cell characteristics work best for each application.
A lush source
Stem cells can develop into various cell types, which makes them the focus of studies that aim to replace cells that fail because of disease, accident or age. Stem cells taken from embryos are more versatile than other types of stem cells, but their use is controversial. For that reason, researchers have studied stem cells from sources other than embryos, including bone marrow, muscle and blood.
Fat tissue comes from the same embryonic tissue as bone marrow, a traditional stem cell source, so scientists reasoned that fat might contain similar cells. In 2002, UCLA researchers discovered stem cells in human fat. They were surprised to find vast quantities.
Stem cells make up 2 to 10 percent of fat tissue. A cubic centimeter of liposuctioned fat (about one-fifth of a teaspoon) yields 100 times as many stem cells as does the same amount of bone marrow, Tuan says. And fat cells are easy to harvest — much easier than bone marrow. One pound of fat removed from a patient’s abdomen can yield up to 200 million stem cells, a more than adequate supply for treatments.
Why fat produces so many stem cells isn’t clear, but Rubin points out that fat tissue serves several important functions. In addition to storing and releasing energy, it helps insulate and protect the body’s internal organs. “Like most tissues in the body, fat has a reservoir of stem cells to replenish cells as they die off or create new cells in response to growth or the need for more cells,” he says.
Fat produces so many stem cells, in fact, that for some applications — such as tissue-replacement or “fat grafting” — there’s no need to grow more of them in the lab. Once harvested, liposuctioned material is treated with enzymes to remove cells from the surrounding tissue, then put into a centrifuge to separate the stem cells from other cell types. In about an hour, the stem cells are ready to be injected back into the patient to plump skin or round out fat tissue lost to injury or disease. Rubin has used this method to treat patients who have lost tissue during breast cancer surgery or have been injured in war. His lab is conducting a clinical trial on the use of fat stem cells to plump up tissue at the site of an amputation to improve the comfort and fit of a prosthetic arm or leg or to make it easier to tolerate sitting for long periods in a wheelchair.
Already, Rubin’s team has treated five military patients, extracting fat from each patient’s abdomen and injecting the stem cells back into the patient at the injury site. He and other scientists think that the fat stem cells remodel tissue by releasing growth factors and communicating with surrounding cells in their new location — sending and receiving signals through chemical cues. As a result, the stem cells enhance the growth of new fat tissue and boost blood supply to surrounding tissue. Over a period of several weeks, the cells he injects form a mound of fat tissue, allowing patients to fit a prosthesis or sit without pain. So far, all of the patients have benefited from the stem cell injections, he says, though his group is still working on how much to inject for each patient. Read full peer reviewed article
Stem cells from fat outperform those from bone marrow in fighting disease
Durham, NC — A new study appearing in the current issue of STEM CELLS Translational Medicine indicates that stem cells harvested from fat (adipose) are more potent than those collected from bone marrow in helping to modulate the body’s immune system. The finding could have significant implications in developing new stem-cell-based therapies, as adipose tissue-derived stem cells (AT-SCs) are far more plentiful in the body than those found in bone marrow and can be collected from waste material from liposuction procedures. Stem cells are considered potential therapies for a range of conditions, from enhancing skin graft survival to treating inflammatory bowel disease. Researchers at the Leiden University Medical Center’s Department of Immunohematology and Blood Transfusion in Leiden, The Netherlands, led by Helene Roelofs, Ph.D., conducted the study. They were seeking an alternative to bone marrow for stem cell therapies because of the low number of stem cells available in marrow and also because harvesting them involves an invasive procedure. “Adipose tissue is an interesting alternative since it contains approximately a 500-fold higher frequency of stem cells and tissue collection is simple,” Dr. Roelofs said. “Moreover,” Dr. Sara M. Melief added, “400,000 liposuctions a year are performed in the U.S. alone, where the aspirated adipose tissue is regarded as waste and could be collected without any additional burden or risk for the donor.” For the study, the team used stem cells collected from the bone marrow and fat tissue of age-matched donors. They compared the cells’ ability to regulate the immune system in vitro and found that the two performed similarly, although it took a smaller dose for the AT-SCs to achieve the same effect on the immune cells. When it came to secreting cytokines — the cell signaling molecules that regulate the immune system — the AT-SCs also outperformed the bone marrow-derived cells. “This all adds up to make AT-SC a good alternative to bone marrow stem cells for developing new therapies,” Dr. Roelofs concluded. “Cells from bone marrow and from fat were equivalent in terms of their potential to differentiate into multiple cell types,” said Anthony Atala, M.D., editor of STEM CELLS Translational Medicine and director of Wake Forest Institute for Regenerative Medicine. “The fact that the cells from fat tissue seem to be more potent at suppressing the immune system suggest their promise in clinical therapies.” Read full peer reviewed article


